Let's Connect!
Sign up to receive exclusive communications about offerings, events, news, surveys, special offers, and related topics via telephone, email and other forms of electronic communications.
Based on the United States Department of Agriculture (USDA) Economic Research Service estimates, about 43 billion kilograms of food, or more than a quarter of the 161 billion kilograms of edible food available for human consumption in the US, were discarded due to spoilage.
Food spoilage is a rising economic and societal concern. It can be defined as a disagreeable change in a food’s standard state that can be perceived by smell, taste, touch, or sight. In other words, spoilage is the process in which food depreciates to the point in which it is not edible, or the level of edibility reduces. These changes are due to many reasons: air and oxygen, moisture, light, microbial growth and temperature. These changes and food spoilage can be minimized by controlling the key component of all foods - water.
Water makes up about 70 percent or more of the weight of most fresh and unprocessed food. Fresh fruits and vegetables contain the most water - between 90-95 percent water. Even ‘‘dry’’ foods like beans, flour and cereals have a common water content of about 10 percent. The determining factor in avoiding spoilage is to accurately measure and monitor the water content of food in the production process, storage and quality control. The amount of water in food (known as percent water) influences the appearance, texture, and flavor of the food. In combination with water activity, the ratio of the vapor pressure of water in a substance to pure water at that temperature also influences shelf life.
Water Content
Water content is an essential and commonly measured attribute of food. Water is vital in food preservation and for a variety of reasons, such as the following:
Since the 1990s, Karl Fischer Titration (KFT) has been the most widely adopted technique for water content determination. Although there are several testing methods available, KFT is the fastest, most accurate, and reliable methods used across industries. Superior accuracy aside, it has gained popularity due to several practical advantages over other methods, such as increased speed, selectivity for water and adaptability to different sample types.
KFT has significant advantages over conventional drying techniques, especially useful when testing the food. When food samples are heated and dried, this can generate additional water due to side reactions between amino acids and reducing sugars (the Maillard reaction) or more commonly results in decomposition of the sample itself. Besides, volatile components contained in the food samples also evaporate and bias the loss on drying result. KFT selectively measures only water contained in a sample, negating these issues, making this method the most accurate and reliable for food processing and production businesses.
The reagent used also has an impact on the effectiveness of the process and the results it delivers. A quality reagent will provide high accuracy, i.e., a stable titer value throughout the reagent’s shelf life in addition to reliable lot-to-lot consistency. Further, as many experienced KFT users know, the method and reagents often need to be modified to solve industry-specific challenges, with technical customer support being a core competency for any reputed supplier.
For example, scientists are often unable to accurately perform KFT on many food samples because water doesn’t exist freely, even though it is their method of choice. Reputed manufacturers troubleshoot such queries daily, even testing samples for customers at their laboratories and providing custom solutions to adjust the method for accurate results.
Challenges in Water Types
Since water doesn’t always exist freely in food samples, it can be categorized into:
Bulk water. Bulk water is free from any other constituents so that each water molecule is surrounded only by other water molecules. It has the same properties as that of water.
Bulk water is the water that can be squeezed out of a food and is easy to measure through titration due to its ability to react with iodine; however, the other types of water prove to be more challenging. Such water needs first to be released by crushing and dissolving samples in solubilizing agents. Solubilizers are recommended based on the chemical food groups (Table 1).
An example of such a sample that often presents challenges due to its limited solubility is chocolate. The very high fat content of chocolate requires the addition of chloroform to dissolve the fat and quickly disperse the chocolate mass. Chocolate also contains a high level of sugar, which dissolves very slowly in alcoholic media. The sugar precipitates during the titration and covers the indication electrode leading to long titration times and incorrect results. For this reason, the addition of formamide is recommended together with chloroform, which dissolves the sugar enabling the electrode to function correctly. 1-hexanol can also be used instead of chloroform as a less toxic alternative (Method details in Lab Report L 060, L 071, L 079, L 028).
By observing the titration curves before and after the addition of solubilizers, we can understand the measurable impact on titration times. In chocolate, a titration in alcoholic media without solubilizers takes four times as long than with solubilizers.
In cases where solubilizers may not result in quick water release, titration at elevated temperatures and addition of homogenizers is recommended. Titration at 40-50C makes for better dissolution; however, this requires a special apparatus (double-walled cells) to do so. Homogenizers can also be used in addition to solubilizers and heat in volumetric cells. The homogenizing device can be mounted on the titrator, and its high-speed stirring action enables better dissolution, shortening analysis time.
In cases where samples are highly insoluble and release water very slowly or have a very inhomogeneous water distribution, external extraction, or dissolution with a solvent is considered. Essentially, the sample is finely crushed and added to a solvent with low water content such as methanol, chloroform, or formamide and is left to stand until the water has been released from the sample. Shaking the solution, keeping it an ultrasonic bath or using a homogenizer can speed up the process.
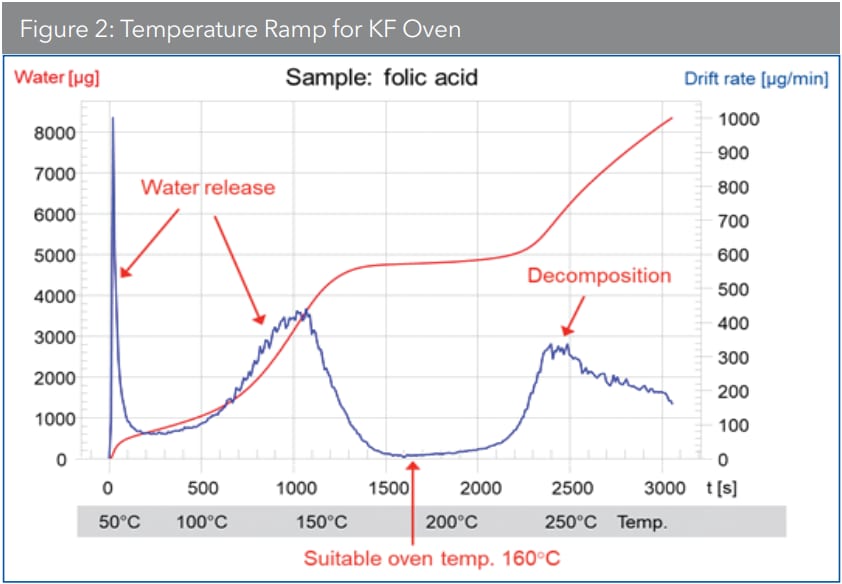
For substances that cause side reactions with KF reagents, however, none of the above are suitable methods. Instead, the sample is heated in a drying oven where the water is vaporized and transferred to a titration cell for titration. It is critical to identify the ideal heating temperature for the sample in this technique since the sample water should be released as fast as possible. Decomposition of the sample, however, must be avoided (Figure 2).
Ensuring Reliable Performance
Often, problems arise as a result of easily correctable issues, such as the wrong choice of methods and incorrect calibration. Regular system care and confirming instrument accuracy through validation by using water standards are also overlooked, which is critical to consistent and reliable performance. This trend is due to KFT not being typically taught in universities. Hence, we often see chemists (and even non-chemists in some cases) learning the method on the job which results in the titration being more error-prone. While SOPs are helpful in such situations, they don’t necessarily prevent errors caused by the lack of knowledge behind the method, which leads to inaccurate results.
While the technique - volumetry or coulometry – is often chosen based on the range of water content to be measured, it is also important to take sample properties, particularly sample size, into account. The sample size for a coulometric determination should be such that it contains approximately 100-5000 μg H2O to achieve a high degree of accuracy. In volumetry, the titrant volume used should ideally be 50 percent of burette volume.
Instrument maintenance and regular verification of results using water standards are also crucial to ensuring reliable performance. To prove the great advantage of water standards over pure water, Honeywell compared titer determination results for two most often used reagents: Hydranal-Composite 5 and Hydranal-Composite 2. As can be observed from our results, using pure water for titer determination leads to a significantly higher standard deviation and therefore to increased uncertainty. Liquid Hydranal-Water Standards are highly recommended for very accurate and precise titer determinations.
Correct KFT implementation will, of course, allow food producers to optimize their processes while retaining international standards of accuracy. But the key is to think bigger. By choosing the right chemical production partner, food and beverage companies have access to a onestop shop for the best possible support throughout their production line, thereby reducing the downstream impact of food spoilage.