Let's Connect!
Sign up to receive exclusive communications about offerings, events, news, surveys, special offers, and related topics via telephone, email and other forms of electronic communications.


(2-[(2,3,4-Trihydroxy)-phenyl]hydrazide · HCl)
HYDRANAL™ Laboratory Report L 416 Rev. 3
This substance causes multiple errors during the standard method of the KF titration:
The end-point of the titration is therefore accidental (Fig. 1), the results are scattered and far too high. Additionally, the sample dissolves poorly in methanol and even worse in ethanol.
The above-mentioned disruptions can be suppressed if the correct quantity of salicylic acid is added to the methanol in the titration vessel. The solubility can be improved by the addition of formamide to the methanol solution (1:1) (Fig. 2).
In our sample (0.5 g) a water content of 1.1 % was determined.1
The indirect method with a Karl Fischer oven in combination with the coulometric determination is not suitable because water release is inhibited. The decomposition of the material starts before all water was released. This reaction cannot be avoided even if nitrogen gas is used as carrier gas (Fig. 3 and Fig. 4).
Procedure for volumetric one-component titration:
Add 30 mL Hydranal-Methanol dry (or Hydranal-Methanol Rapid), 30 mL Hydranal-Formamide dry and 7 g Hydranal-Salicylic acid to the titration vessel and titrate to dryness with Hydranal-Composite 5. Precisely weigh-in a 0.5 g sample using differential weighing and titrate the water content with Hydranal-Composite 5.
Please note: Mixtures of alcohol and formamide should be prepared freshly every day. If the mixture is stored longer than 2 days, undesirable side reactions can occur.
Alternatively to the use of salicylic acid, it is also possible to use 40 mL Hydranal-Buffer for Bases and 20 mL Hydranal-Formamide Dry. However, the solubility of the sample is decreased in that medium. To ensure a full dissolution of the sample, longer extraction times should be chosen (manual programming).
1 In dependence on the manufacturer the water content can vary widely (0 – 3% H2O).
Procedure for volumetric two-component titration:
For this substance the titration must be carried out with the reagents specified in the method. The two component reagent system Hydranal-Titrant / Hydranal-Solvent cannot be used since the interference reaction cannot be fully suppressed in this case.
Procedure for coulometric titration with KF oven:
Not applicable, see introduction
Note: A Suitability test including protocol according to Ph. Eur. is available on request (hydranal@honeywell.com).
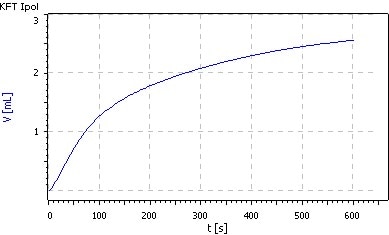
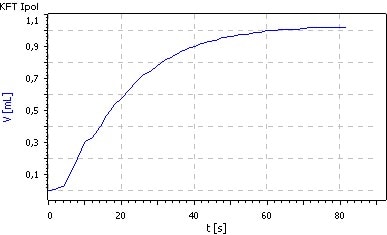
Fig. 1. Titration of 0.5 g benserazide
hydrochloride in 30 mL Hydranal-
Methanol Dry with Hydranal-
Composite 2 . No stable end point is reached
Fig. 2. Titration of 0.5 g benserazide
hydrochloride in 30 mL Hydranal-
Methanol Dry, 30 mL Hydranal-
Formamide Dry and 7 g salicylic acid with Hydranal-Composite 5
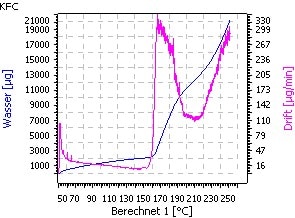
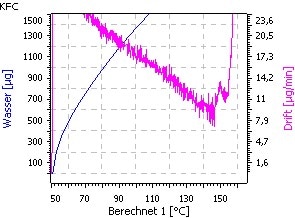
| Fig. 3. Temperature ramp: 50 − 250°C; 1°C/min | Fig.4. Zoom- Transition: water release/decomposition |